Adapted Test Protocol
Day 1
- Cells in glycerol stock (- 80 °C) are transferred onto agar plate with an inoculation loop and incubated at 35 °C for 16h – 24h.
 Day 2
Day 2
- Autoclave 500 mL TSB and 500 mL TSB-Agar at 121 °C for 20 minutes. Prepare at least 10 agar plates in the safety cabinet. In the safety cabinet by keeping covers open, allow steam to leave petri dishes.
- Prepare 3 samples treated with antibacterial agent and 6 samples without antibacterial treatment with an area of 25mm x 25mm.
- Put 3 mL TSB into 3 different sterile 17x100mm test tubes and transfer 1 loop of bacterial colony into two of them. Use third tube as negative control group.

- Incubate tubes at 37 °C with 220 RPM shaking for 18h – 20h.
Day 3
- Dip parafilm pieces in 20x20mm size into 70% ethanol for sterilization in the cabinet. Before employing, take them out of ethanol and let them dry.
- Prepare water containers to provide humidity in the incubator.
- Centrifuge cells in one of the test tube at 5000 RPM for 4 minutes and replace supernatant with 3 mL fresh 1/500 X TSB.
- Measure OD600 values of cells in 3 mL 1/500x TSB. To do so, dilute cells 10 times by mixing 150 µL of cells and 1350 µL distilled water.
- Calculate OD600 value of cells in 3 mL (main stock) and dilute cells with distilled water in a final volume of 1 mL with fresh 1/500 X TSB to obtain OD600 value of 0.001 (for S.aureus RN4220 when OD600 = 0.6; there are 5×108 CFU/mL). Mind that the bacteria concentration inoculated on the test piece shall be 6.2×103 to 2.5×104 cells/cm2 or 2.5×105 to 10×105 cells/mL.
- Take agar plates out of fridge and allow them to reach room temperature.
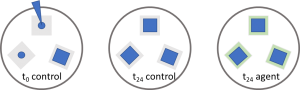
- Put test materials into sterile petri dishes as triple. 3 x (t0 control, t24 control, t24 agent)
- Drop 50 to 100max µL of cells onto test materials and cover them with sterilized parafilm.
- Put one cap of water into each petri dish to provide extra humidity.
- Take t0 control group test materials into falcon tubes containing 20 mL TRIS pH 7.6 solution. Vortex falcon tubes for 1 minute and make sure that parafilm is removed from test material.

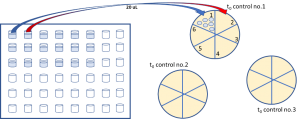
- For serial dilution, get a 96-well plate and put 270 µL TRIS solution into wells. Dilute vortexed solution in 96-well plates by adding 30 µL of cells into first well. Continue serial diluting by adding each well 30 µL of cells from the previous well. (6 times dilution would be enough)
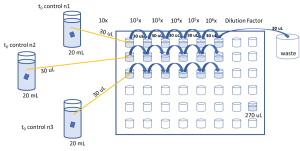
- With a pen, slice agar plates into six zones and transfer 20 µL of cells from each well into each zone. Allow solution to dry and later inoculate plates overnight at 35 °C.
Day 4
- Take t0 control group from incubator and start counting.
- From serial dilutions, zones having 30 to 300 colonies are used in calculations to determine colony number in the main stock.
- Take 6 agar plates out of fridge and allow them to reach room temperature.
- Take t24 control and t24 agent group test materials into falcon tubes containing 20 mL TRIS pH 7.6 solution. Vortex falcon tubes for 1 minute and make sure that parafilm is removed from test material.

- For serial dilution, get a 96-well plate and put 270 µL TRIS solution into wells. Dilute vortexed solution in 96-well plates by adding 30 µL of cells into first well. Continue serial diluting by adding each well 30 µL of cells from the previous well. (6 times dilution would be enough.)
- With a pen, slice agar plates into six zones and transfer 20 µL of cells from each well into each zone. Allow solution to dry and later inoculate plates overnight at 35 °C.

Day 5
- Take t24 control and t24 agent group plates from incubator and start counting.
- From serial dilutions, zones having 30 to 300 colonies are used in calculations to determine colony number in the main stock.
- t0 control and t24 control groups are expected to have similar number of colonies while t24 agent is much less than them in case it has antibacterial property. Calculate percentage of decrease in colony number.